Glaucoma
Immunological Mechanisms in Glaucoma
Glaucoma, one of the leading causes of blindness worldwide 1 , represents a group of ocular disorders that are responsible for loss of retinal ganglion cells and gradual loss of visual field. People of all ages can be affected by this disease. At age 70, about 7% of the population are suffering from glaucoma 2 . Currently, the most important screening methods for glaucoma patients include tonometry, ophthalmoscopy, and perimetry. Today, it is commonly accepted that glaucoma becomes clinically detectable about 10 years after onset. More than 500.000 people are suffering from undiagnosed glaucoma in Germany. Furthermore, about 8-10% of patients at the age of 40 have elevated intraocular pressure (IOP). Only some of them will convert to glaucoma and will therefore need treatment. With the clinical methods available today, it is not possible to decide who needs to be treated. Because only 76% of glaucoma patients have an IOP higher than 21 mm Hg and additionally IOP of about 16% of glaucomatous eyes was never recorded above 21 mm Hg 3 , a more objective and more sensitive diagnostic method, which is independent from IOP, is needed. An earlier detection of glaucoma would result in an earlier treatment, which can prevent blindness or slow down the disease process significantly.
Over 10 years ago Wax et al. first reported an elevated antibody reactivity in patients with NTG 10, 11 demonstrating an increased level of heat shock protein 60 (HSP60) antibodies 10 . During the next years this research group and others found several serum autoantibodies that are elevated in glaucoma patients, e.g. higher levels of antibodies against small heat shock proteins (αA-, αB-crystalline, and HSP27) were identified in NTG patients 12 . To gain more insight into the role of heat shock proteins in glaucoma Tezel et al. examined the pathogenetic effects of antibodies against small heat shock proteins on isolated human retinae. They could show that direct application of antibodies against αA- and αB-crystalline or HSP27 results in apoptosis of neurons and cells of the retinal vasculature 12 . Increased expression of HSP27 and HSP60 were observed in retina and optic nerve head in human donor eyes with glaucoma 13 .
Several further serum antibodies were detected in glaucoma patients like gamma-enolase 14 , glutathione-S-transferase 15 , antiphosphatidylserine 16 , neuron specific enolase 17 , and glycosaminoglycans 18 .
It is known that complex natural autoantibody profiles exist even in healthy people 19, 20 , hence it can be important not to screen only for one or a few antibody reactivities, but to investigate disease specific changes in complex profiles of naturally occurring autoantibodies.
The molecular specificity of the body is reflected in sets of anti-self receptors of autoreactive T-lymphocytes and natural autoantibodies, the totality of which forms the “immunological homunculus 21, 22 . This natural autoantibody repertoire is thought to be very stable in healthy subjects during long lasting live periods.
These natural autoantibodies can be considered as regulatory factors 23 , potentially able to modulate the activity of target molecules and influence their physiological functions. Therefore, the understanding of the role of up- and downregulation of autoantibodies is very complex. To give an example, although inhibition of the growth of neuronal processes by anti-NGF antibodies has been demonstrated 24 , a combination of antibodies directed against different epitopes of NGF receptors results in a range of effects from mainly trophic influence up to the induction of neuronal differentiation 25 . Other regulatory effects of autoantibodies include their ability to activate an array of receptors in living cells, thereby increasing the production of the secondary messengers and inducing complex cascades 23, 26, 27 .
This complex autoantibody repertoire in healthy subjects can be considered as the “gold-standard” where we attempted to compare some disease associated patterns to. However, the analysis of these repertoires is hampered by the fact that those immune reactivities need to be filtered from the “immunological noise” which are correlated to the disease state.
Our group developed several approaches to screen those antibody repertoires which are based on Western Blotting and on bead based mass spectrometry 7, 28-31 .
In those profiling experiments, the ocular antigens were separated by gel electrophoresis according to their molecular weight and transferred to nitrocellulose membrane by western blotting. These immobilized antigens were incubated with the sera of patients (containing the autoantibodies) and the immunreactions were visualized by staining reaction. The antibody patterns were digitized and subsequently analyzed by pattern matching algorithms such as artificial neural networks and multivariate statistics.
In several studies we could prove that complex antibody profiles exist in glaucoma patients that are significantly different from antibody profiles in healthy people 7-9, 31, 32 . We also performed studies using human antigens to confirm that these antibodies are really autoantibodies 4, 33 .
With antibody profiling techniques which were based on Western blotting and digital image analysis combined with multivariate statistics and artificial neural networks, we detected significant antibody patterns against ocular antigens in sera of glaucoma patients 7, 9, 34 , and identified antibody reactivities against e.g. alpha-fodrin 7 , cellular retinaldehyde-binding protein 4 , retinal S-antigen 4 , HSP27 9 , or HSP70 35 .
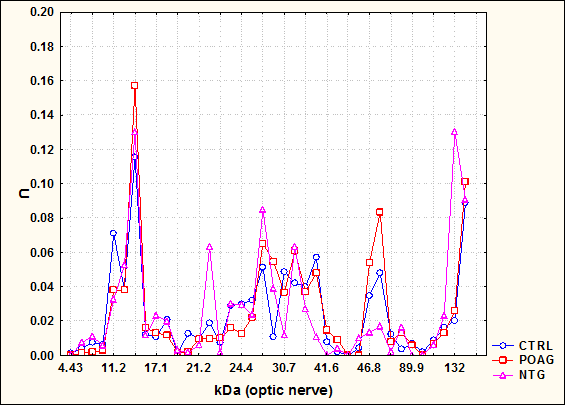
Figure 1: This graph shows antibody profiles in 2 glaucoma groups (POAG and NTG) in comparison to healthy controls (CTRL). The x-axis shows the molecular weight in Kilodalton (kDa) and the y-axis the antibody reactivity (U). Complex antibody profiles were detected in all groups. The antibody profiles of the glaucoma groups were significantly different from controls. (Mol Vis 2004)
Furthermore we could demonstrate that these complex antibody profiles do exist amongst different study populations 7 . Figure 2 displays IgG antibody patterns in NTG patients in study populations from Germany and the United States. In this figure not only increased antibody reactivities in the glaucoma groups could be in comparison to controls but also vast and overlapping areas of down-regulations between both study populations. An enhanced antibody reactivity to alpha-fodrin (120 kDa), one of the major neuronal cytoskeleton proteins, could be observed NTG sera. The presence of alpha-fodrin antibodies was confirmed by ELISA, in which a highly elevated anti-alpha-fodrin titer was found in NTG patients in comparison to control subjects (P<0.01) or POAG patients (P<0.04). In Alzheimer’s disease, an anti-brain spectrin (=fodrin) immunoreactivity could be demonstrated 36-38 . Furthermore, alpha-fodrin is a target of caspase-3 and is cleaved by caspases at very early stages of apoptosis leading to structural rearrangements including membrane blebbing 39 . This result provides hints for an implication of caspase activation in glaucoma patients. This is in accordance with other studies who demonstrated that alpha-fodrin is cleaved by caspase3 in a chronic ocular hypertensive rat model of glaucoma 40, 41 . The elevated immunreactivity against fodrin provides evidence that there might be shared mechanisms in the pathogenesis of glaucoma and other neurodegenerative diseases like Alzheimer’s.
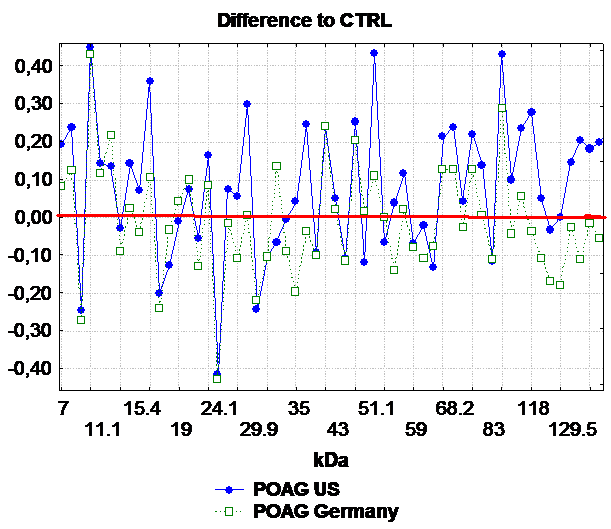
Figure 2: Comparison of the mean antigen-antibody-reactivity of patients with primary open-angle glaucoma (POAG) from the US study population (=blue) to the German (=green). The differences to the control group in the mean antigen-antibody reactivity were plotted against the corresponding molecular weight of the optic nerve antigen. The x-axis is the molecular weight (kDa) and the y-axis the difference in the density of the antigen-antibody reaction compared to controls (U). All reactivities above zero (red line) represent up-regulations in comparison to healthy subjects, all below zero down-regulations in the comparison to the control group (=red line). (IOVS 2006).
However, Western blot technique is very time consuming, reproducibility is difficult, and therefore not really suitable for clinical large scale applications. Therefore, we developed a ProteinChip approach to find a faster screening method not relying on Western blotting 32, 42, 43 . Mass spectrometry measurements are also more accurate regarding the molecular weight of the peaks than Western blots and are much easier to use, especially since multiple antibody reactivities need to be analyzed in glaucoma patients.
Most previous antibody profiling studies were performed using a modified Western blotting technique combined with subsequent digital image analysis and multivariate statistics. Due to large numbers of antibody reactions that have to be screened simultaneously, it is a very tedious and time-consuming process to achieve high precision in determining molecular weights and in reproducibility. Furthermore, we compared both techniques and found a very much higher sensitivity in the ProteinChip mass spectrometric approach 31 .
Both techniques in our studies have in common that we could confirm known up-regulations of immunoreactivies in glaucoma, but most interestingly we could furthermore demonstrate some regions with down-regulated immunoreactivities in glaucoma patients in comparison to healthy subjects. Figure 1 shows complex antibody profiles against optic nerve antigens in sera of POAG, NTG, in comparison to healthy controls (CTRL). Several molecular weight regions with elevated antibody reactivity in the NTG group can be seen, e.g. 22, 27, or 75 kDa. However, several regions with lower reactivities could be found in this group in comparison to all other groups, e.g. at approx. 39 kDa 9 . In terms of statistical distances, the NTG group revealed to be the most different from all other groups in this study (P<0.002; distance NTG-CTRL=5.76).
A condition where antibody- and cell-based immunreactions against structures of the own body occur is considered an autoimmune disease. In autoimmune diseases aggressive and elevated circulating autoreactive antibodies develop and react against self-antigens 35 .
Elevated autoantibodies are found in classical autoimmune diseases, e. g. in patients suffering from systemic Lupus erythematosus, primary antiphospholipid syndrome 36 , and primary Sjörgen’s syndrome 37 . Myasthenia gravis patients have antibodies against muscular acetycholin receptors 38, 39 . But even in typical autoimmune diseases like myasthenia gravis there are some patients that suffer from the disease and do not have antibodies against acetycholin receptors 40 . Antibodies also do not occur in all patients with glaucoma, e.g. Maruyama et al. detected antibodies against gamma-enolase in about 25% of glaucoma patients (POAG and NTG) they had included in their study (in comparison to 12% of healthy controls) 19 .
Today, our understanding of the effects of antibody levels that are outside these upper or lower limits, is not well studied, however in certain conditions, it is clear that pathological sequelae will develop. Typically, this is the result of excessive antibody production as in the case of myasthenia gravis 44, 45 . Less well understood is the finding and relevance of decreased autoantibodies, however associations and findings are now beginning to emerge in both the neurologic and diabetic disorders 46, 47 .
Wax and Tezel could demonstrate in multiple studies that there are elevated immunoreactivites in normal pressure glaucoma patients and therefore they proposed an autoimmune involvement in glaucoma. They found especially increased levels of autoantibodies against different heat shock proteins such as HSP 27 and HSP60 and concluded that this might represent a generalized response to tissue stress or damage.
However, this approach would imply that glaucoma is an autoimmune disease in some glaucoma patients e.g. in normal pressure glaucoma.
Our group could reveal specific changes in the natural autoantibody repertoires in glaucoma patients. Whereas the up-regulations of immune-reactions in these autoantibody profiles are in concordance with the findings of Wax and Tezel, our group could demonstrate significant and specific down-regulations of immunoreactivities in glaucoma patients. Furthermore, these antibody patterns are not only specific for normal pressure glaucoma patients, but also for primary open angle glaucoma. These down regulations cannot be explained by mechanisms of classical autoaggressive autoimmune diseases.
In general, the role of these antibody reactions is widely unclear. They can be causative to the disease, but some of them could also develop as a consequence of the disease (epiphenomenon). This question will be addressed in autoimmune Glaucoma Animal models
To understand the down regulations of immunereactivities, one has to bear in mind that the molecular specificity of the body is reflected in sets of anti-self receptors of autoreactive T-Lymphocytes and natural autoantibodies, the totality of which forms the “immunological homunculs” 62, 63 .
Furthermore, it is important to consider that antibody cannot have only destructive functions, but are also regulatory factors 64-66 .
In glaucoma, we hypothesize that the loss of some endogenous naturally occurring and maybe protective autoantibodies may lead to a loss of immune protection or protective factors and thus to an increased risk to develop the disease.
If this loss of protective effects of natural autoimmunity leads to the development of glaucoma or to an increased risk to develop the disease, this might lead to innovative new therapeutic immunomodulatory strategies. This is in accordance with the speculative hypothesis of Schwartz and colleagues who attempt to boost natural ”protective” autoimmunity for therapeutic gain in patients with glaucoma 72 . In contrast, considering these vast amounts of lowered immunoreactivites we found in our studies in glaucoma patients, we would not expect that e.g. a treatment by plasmapheresis as in classical autoimmune diseases might be promising in glaucoma.
Taking all these findings together, we emphasize that there is strong evidence for the involvement of autoimmune reactions in glaucoma.
Recent publications:
- Beutgen VM, Pfeiffer N, Grus FH (2021) Serological Levels of Anti-clathrin Antibodies Are Decreased in Patients With Pseudoexfoliation Glaucoma. Frontiers in immunology 12:616421.
- Beutgen VM, Schmelter C, Pfeiffer N, Grus FH (2020) Autoantigens in the trabecular meshwork and glaucoma-specific alterations in the natural autoantibody repertoire. Clin Transl Immunology 9:e01101.
- Liu H, Anders F, Funke S, Mercieca K, Grus F, Prokosch V (2020) Proteome alterations in aqueous humour of primary open angle glaucoma patients. International journal of ophthalmology 13:176-179.
- Schmelter C, Fomo KN, Perumal N, Manicam C, Bell K, Pfeiffer N, Grus FH (2019) Synthetic Polyclonal-Derived CDR Peptides as an Innovative Strategy in Glaucoma Therapy. J Clin Med 8.
- Beutgen VM, Perumal N, Pfeiffer N, Grus FH (2019) Autoantibody Biomarker Discovery in Primary Open Angle Glaucoma Using Serological Proteome Analysis (SERPA). Front Immunol 10:381.
- Bell K, Funke S, Grus FH (2019) [Autoimmunity and glaucoma]. Ophthalmologe 116:18-27.
- Bell K, Und Hohenstein-Blaul NVT, Teister J, Grus F (2018) Modulation of the Immune System for the Treatment of Glaucoma. Curr Neuropharmacol 16:942-958.
- Schmelter C, Perumal N, Funke S, Bell K, Pfeiffer N, Grus FH (2017) Peptides of the variable IgG domain as potential biomarker candidates in primary open-angle glaucoma (POAG). Hum Mol Genet 26:4451-4464.
References:
1. Quigley HA. Number of people with glaucoma worldwide. Br J Ophthalmol 1996;80:389-393.
2. Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol 2006;90:262-267.
3. Sommer A, Tielsch JM, Katz J, et al. Relationship between intraocular pressure and primary open angle glaucoma among white and black Americans. The Baltimore Eye Survey. Arch Ophthalmol 1991;109:1090-1095.
4. Reichelt J, Joachim SC, Pfeiffer N, Grus FH. Analysis of autoantibodies against human retinal antigens in sera of patients with glaucoma and ocular hypertension. Archives of Ophthalmology submitted 2006.
5. Joachim SC, Pfeiffer N, Grus FH. Detection of IgG antibody patterns against retinal antigen in aqueous humor of patients with normal tension glaucoma. Experimental Eye Research submitted 2004.
6. Storf J, Thiel U, Pfeiffer N, Grus FH. Characterization of changes in antibody patterns in glaucoma patients by means of protein-microarray-technology. DOG Meeting 2006.
7. Grus FH, Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Wax MB. Serum Autoantibodies to {alpha}-Fodrin Are Present in Glaucoma Patients from Germany and the United States. Invest Ophthalmol Vis Sci 2006;47:968-976.
8. Joachim SC, Pfeiffer N, Grus FH. Autoantibodies in patients with glaucoma: a comparison of IgG serum antibodies against retinal, optic nerve, and optic nerve head antigens. Graefes Arch Clin Exp Ophthalmol 2005;243:817-823.
9. Grus FH, Joachim SC, Hoffmann EM, Pfeiffer N. Complex autoantibody repertoires in patients with glaucoma. Mol Vis 2004;10:132-137.
10. Wax MB, Tezel G, Saito I, et al. Anti-Ro/SS-A positivity and heat shock protein antibodies in patients with normal-pressure glaucoma. Am J Ophthalmol 1998;125:145-157.
11. Wax MB, Barrett DA, Pestronk A. Increased incidence of paraproteinemia and autoantibodies in patients with normal-pressure glaucoma. Am J Ophthalmol 1994;117:561-568.
12. Tezel G, Seigel GM, Wax MB. Autoantibodies to small heat shock proteins in glaucoma. Invest Ophthalmol Vis Sci 1998;39:2277-2287.
13. Tezel G, Hernandez R, Wax MB. Immunostaining of heat shock proteins in the retina and optic nerve head of normal and glaucomatous eyes. Arch Ophthalmol 2000;118:511-518.
14. Maruyama I, Ohguro H, Ikeda Y. Retinal ganglion cells recognized by serum autoantibody against gamma-enolase found in glaucoma patients. Invest Ophthalmol Vis Sci 2000;41:1657-1665.
15. Yang J, Tezel G, Patil RV, Romano C, Wax MB. Serum autoantibody against glutathione S-transferase in patients with glaucoma. Invest Ophthalmol Vis Sci 2001;42:1273-1276.
16. Kremmer S, Kreuzfelder E, Klein R, et al. Antiphosphatidylserine antibodies are elevated in normal tension glaucoma. Clin Exp Immunol 2001;125:211-215.
17. Ikeda Y, Maruyama I, Nakazawa M, Ohguro H. Clinical significance of serum antibody against neuron-specific enolase in glaucoma patients. Jpn J Ophthalmol 2002;46:13-17.
18. Tezel G, Edward DP, Wax MB. Serum autoantibodies to optic nerve head glycosaminoglycans in patients with glaucoma. Arch Ophthalmol 1999;117:917-924.
19. Li WH, Zhao J, Li HY, et al. Proteomics-based identification of autoantibodies in the sera of healthy Chinese individuals from Beijing. Proteomics 2006;6:4781-4789.
20. Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today 1991;12:154-159.
21. Nobrega A, Haury M, Grandien A, Malanchere E, Sundblad A, Coutinho A. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological "homunculus" of antibodies in normal serum. Eur J Immunol 1993;23:2851-2859.
22. Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunology Today 1991;1991:154-159.
23. Poletaev A, Osipenko L. General network of natural autoantibodies as immunological homunculus (Immunculus). Autoimmun Rev 2003;2:264-271.
24. Johnson EM, Jr., Gorin PD, Osborne PA, Rydel RE, Pearson J. Effects of autoimmune NGF deprivation in the adult rabbit and offspring. Brain Res 1982;240:131-140.
25. Poletaev AB, Abrosimova AA, Sokolov MA, et al. Dialectics and implications of natural neurotropic autoantibodies in neurological disease and rehabilitation. Clin Dev Immunol 2004;11:151-156.
26. Kulberg AY. Immunoglobulins and Antibodies. Russ J Immunol 1999;4:241-242.
27. Erlanger BF. Some thoughts on the structural basis of internal imagery. Immunol Today 1989;10:151-152.
28. Joachim SC, Wuenschig D, Pfeiffer N, Grus FH. IgG antibody patterns in aqueous humor of patients with primary open angle glaucoma and pseudoexfoliation glaucoma. Mol Vis 2007;13:1573-1579.
29. Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Antibodies to alpha B-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor. Curr Eye Res 2007;32:501-509.
30. Joachim SC, Bruns K, Lackner KJ, Pfeiffer N, Grus FH. Analysis of IgG antibody patterns against retinal antigens and antibodies to alpha-crystallin, GFAP, and alpha-enolase in sera of patients with "wet" age-related macular degeneration. Graefes Arch Clin Exp Ophthalmol 2007;245:619-626.
31. Grus FH, Joachim SC, Pfeiffer N. Analysis of complex autoantibody repertoires by surface-enhanced laser desorption/ionization-time of flight mass spectrometry. Proteomics 2003;3:957-961.
32. Singer HS, Loiselle CR, Lee O, Garvey MA, Grus FH. Anti-basal ganglia antibody abnormalities in Sydenham chorea. J Neuroimmunol 2003;136:154-161.
33. Joachim SC, Reichelt J, Pfeiffer N, Grus FH. Autoantibody profiles against human retina in sera of glaucoma patients. DOG Meeting 2006.
34. Joachim SC, Grus FH, Pfeiffer N. Analysis of autoantibody repertoires in sera of patients with glaucoma. Eur J Ophthalmol 2003;13:752-758.
35. Joachim SC, Bruns K, Lackner K, Pfeiffer N, Grus FH. Antibodies to αB-crystallin, vimentin, and heat shock protein 70 in aqueous humor of patients with normal tension glaucoma and IgG antibody patterns against retinal antigen in aqueous humor Experimental Eye Research submitted 2004.
36. Fernandez-Shaw C, Marina A, Cazorla P, Valdivieso F, Vazquez J. Anti-brain spectrin immunoreactivity in Alzheimer's disease: degradation of spectrin in an animal model of cholinergic degeneration. J Neuroimmunol 1997;77:91-98.
37. Sihag RK, Cataldo AM. Brain beta-spectrin is a component of senile plaques in Alzheimer's disease. Brain Res 1996;743:249-257.
38. Vazquez J, Fernandez-Shaw C, Marina A, Haas C, Cacabelos R, Valdivieso F. Antibodies to human brain spectrin in Alzheimer's disease. J Neuroimmunol 1996;68:39-44.
39. Janicke RU, Ng P, Sprengart ML, Porter AG. Caspase-3 is required for alpha-fodrin cleavage but dispensable for cleavage of other death substrates in apoptosis. J Biol Chem 1998;273:15540-15545.
40. McKinnon SJ. Glaucoma: ocular Alzheimer's disease? Front Biosci 2003;8:s1140-1156.
41. Tahzib NG, Ransom NL, Reitsamer HA, McKinnon SJ. Alpha-fodrin is cleaved by caspase-3 in a chronic ocular hypertensive (COH) rat model of glaucoma. Brain Res Bull 2004;62:491-495.
42. Joachim SC, Kraft D, Pfeiffer N, Wax MB, Grus FH. Antibody Profile Analysis via Western Blot and Protein-Chip Methods in a Retinal Ganglion Cell Degeneration Animal Model. HUPO Meeting 2006.
43. Joachim SC, Kraft D, Pfeiffer N, Wax MB, Grus FH. Antibody Profile Analysis via Western Blot and Protein-Chip Methods in a Retinal Ganglion Cell Degeneration Animal Model. From Genome to Proteome Meeting 2006.
44. Zimmermann CW, Eblen F. Repertoires of autoantibodies against homologous eye muscle in ocular and generalized myasthenia gravis differ. Clin Investig 1993;71:445-451.
45. Zimmermann CW, Weiss G. Antibodies not directed against the acetylcholine receptor in myasthenia gravis. An immunoblot study. J Neuroimmunol 1987;16:225-236.
46. Poletaev AB, Morozov SG, Gnedenko BB, Zlunikin VM, Korzhenevskey DA. Serum anti-S100b, anti-GFAP and anti-NGF autoantibodies of IgG class in healthy persons and patients with mental and neurological disorders. Autoimmunity 2000;32:33-38.
47. Poletaev AB, Gnedenko BB, Makarova AA, et al. Possible mechanisms of diabetic fetopathy. Hum Antibodies 2000;9:189-197.
